Measles-Rubella Elimination and Fever-Rash Surveillance
WHO South-East Asia Region (SEAR), including India, resolved in Sept 2019 to eliminate Measles and Rubella by 2023 to prevent deaths and disabilities caused by these highly infectious childhood diseases. Measles is one of the most common vaccine-preventable diseases with high morbidity and mortality among the under-five children in India, though the country has been providing Measles vaccination under UIP since 1985 across all the states. Most Measles related deaths are caused by serious complications like encephalitis, severe diarrhea and related dehydration or severe respiratory infections such as pneumonia. In addition, Rubella transmission is also highly prevalent across the country, which can affect susceptible pregnant mothers and may lead to CRS (Congenital Rubella Syndrome) in the newborn. CRS is a complex of congenital anomalies that can affect multiple organ systems causing spontaneous abortions and still-births as well as lifelong disabilities in a child. Although there is no specific treatment for both Measles and Rubella, these diseases can be very well prevented by immunization with the available highly efficacious and cost-effective MR vaccine.
India, committed to the goal of Measles Rubella elimination by 2023, kick started MR surveillance as a laboratory supported Outbreak-based surveillance in 2005 which expanded to all the States by 2015. India began transitioning to MR case based surveillance in 2016 and expanded to all the states by 2019. Fever and Rash (FR) surveillance, with a broadened case definition, was started in 2018 and expanded to all the States in 2021 .
The key elimination strategies are:-
- Achieve and maintain atleast 95% coverage of both the doses of MRCV (Measles Rubella containing Vaccine) within each district of the country through routine and/or supplementary immunization. India introduced MR (Measles Rubella) vaccine in routine immunization program after a nation-wide MR campaign rolled out in a phased manner, targeting the children aged 9 months to <15 years irrespective of their previous measles/MR vaccination status or history of illness. (Both the doses of measles vaccine in RI (at 9-12 months and 16-24 months) are substituted with MR vaccine).
- Develop and sustain a sensitive case based Fever Rash and CRS surveillance system that fulfills recommended elimination standard surveillance performance indicators
- Measles case management including Vitamin A supplementation to reduce the morbidity, mortality, and the spread of the disease.
- Ensure adequate outbreak preparedness and respond rapidly to Measles and Rubella outbreaks.
FAQs
1. What is Fever-Rash Surveillance for Measles Rubella elimination?
It is the collection of Fever Rash data for action. Under Fever-Rash surveillance, every Fever with Maculopapular Rash case is to be reported, investigated and followed up.
2. What is to be reported in Fever-Rash surveillance?
Irrespective of the age
Any person in whom a clinician suspects Measles or Rubella infection OR
Any person with Fever and Maculopapular rash is to be reported.
3.What is expected of a clinician when he comes across a suspected Measles/Rubella case or a case of Fever with Maculopapular Rash?
Report immediately all the Fever with maculopapular rash cases (irrespective of the clinical diagnosis, associated symptoms/signs, vaccination status, or age) to the Dist. RCH Officer/ SMO office over telephone / SMS / WhatsApp. Note the complete address and contact number so that the case can be tracked and followed up. If possible, support in sample collection.
4.How is a Measles/Rubella or a case of Fever with Maculopapular Rash investigated?
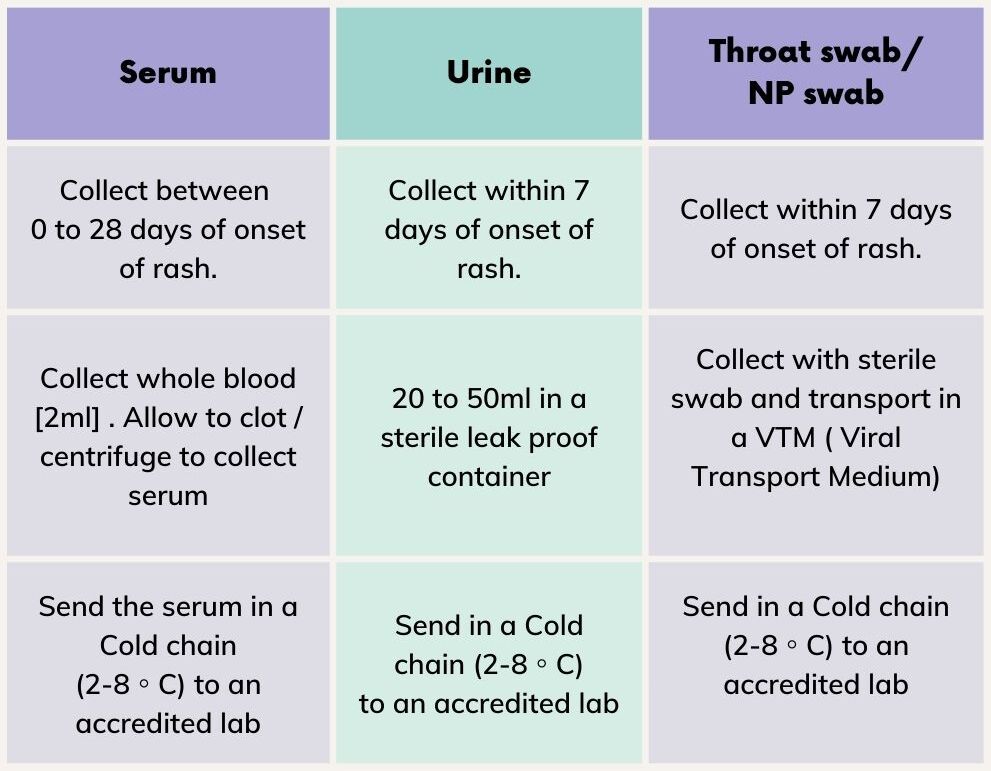
Every Measles/Rubella/Fever with maculopapular rash case reported is clinically examined and a specific Case Investigation Form (CIF) is filled up. Serum and Virology (Throat swab/ NP swab/ Urine) samples are collected from all the eligible cases and are transported in a cold chain (2-8◦C) to NIV-Alappuzha / State PH Lab, Tvpm for Ig M and Virological study.
The lab result (positive or negative) will be available within a week and based on it, the case is classified.
5.What actions are taken after each Measles/Rubella/ Fever with Maculopapular Rash case is reported?
- Case Investigation by MO within 48 hrs and complete the CIF (Case Investigation Form)
- Collect Serum and Virology (Throat swab/ NP swab/ Urine) samples and send them to an accredited lab
- Case management including Vitamin A supplementation
- Community search and sensitization for additional cases/ immunisation status
6.Sample collection
7. How is a suspected Measles /Rubella/ Fever with Maculopapular Rash case classified?
- Laboratory-confirmed Measles/ Rubella: A case that meets the clinical case definition and the lab result (Serum or Virology)confirms Measles/ Rubella.
- Epidemiologically Linked Measles/ Rubella: A case that meets the clinical case definition. But no adequate sample is collected/ tested; but is linked to a laboratory-confirmed case of Measles/Rubella.
- Clinically compatible Measles/ Rubella: A case that meets the clinical case definition. But no adequate sample is collected/ tested; and is not linked to a laboratory-confirmed case of Measles, Rubella or any other communicable disease.
- Discarded as Non Measles Non Rubella exanthem: A suspected case in which adequate samples (including Serum) are tested Negative for both Measles and Rubella by an accredited Lab or a case that does not meet the clinical case definition of Measles/ Rubella or a case that is epidemiologically linked to a Non Measles Non Rubella outbreak or a case epidemiologically linked to another laboratory-confirmed communicable disease.
8. Management of uncomplicated cases
- Give 2 doses of vitamin A as per schedule.
- Control fever with antipyretics ; Provide nutritional support to the child
- Explain to the mother regarding the increased risk of diarrhea, ARI and other infections
- Advise mother to:
- Treat the child at home; Watch for signs of complication; Continue breast feeding/ food to the child ; Give appropriate fluids at frequent intervals ; Seek medical advice if any complications.
9. Complications of Measles [Around 10-15 % cases develop complication]

10. Dose of Vitamin A for Measles cases

11. What is an outbreak of Measles/Rubella?
Five or more suspected Measles/Rubella cases or One or more suspected Measles death from one block / planning area/ contiguous blocks in a month / consecutive 4 weeks is considered as an outbreak.
12. What are the actions taken during a Measles/Rubella outbreak:
A door to door search is conducted in the area for finding more Measles/Rubella cases. Line listing of all the suspected Measles/ Rubella cases/ deaths in the previous 3 months is done. Two doses of Vitamin A is given to all Measles cases. All houses visited are marked (M/date) after a thorough search.
Serum samples are collected from 5 Cases (collected between 0 to 28 days of onset of rash).Virology samples (Urine or Throat/ NP swab) are collected from 2 cases (collected within 7 days of onset of rash). Samples are sent to NIV-Alappuzha / SPH Lab, Tvpm in a cold chain (2-8 C).
13. Classification of outbreak –based on lab of the samples sent

Please Report Immediately any Fever with Maculopapular Rash case to : Dr. PRATHAPA CHANDRAN C , SMO-WHO – 85477 43841